Spirale® Drug Delivery System
Spirale® Drug Delivery System
Spirale® Drug Delivery system (DDS) spacer for Metered Dose Inhalers (MDI) Reduce the risk of pathogen transmission.
Spirale® can help to reduce the risk of pathogen transmission.
In previous outbreaks (SARS, H1N1 and MERS) the World Health Organisation highlighted that Aerosol generating procedures increased risk of pathogen transmission. The Intensive Care society have issued similar advice in their COVID-19 communication stating aerosol generating procedures should be avoided: 2019-nCOV – Critical Care Society advice.
Spirale® drug delivery system is an alternative to nebulisation for bronchodilator therapy.
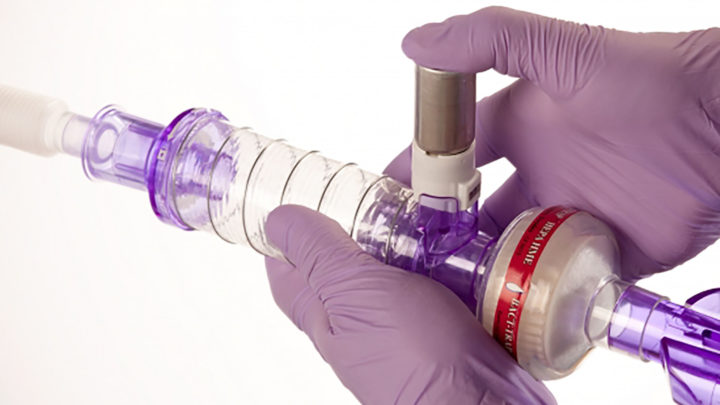
This collapsible volumising chamber is designed to deliver micro-drug particles from a Metered Dose Inhaler (MDI). The product is intended to remain in the breathing circuit, therefore removing the risk of environmental contamination in the clinical setting.
Spirale® DDS optimises alveolar drug deposition using a carefully designed jet orifice in combination with volumising geometry.
Invasive Ventilation
Spirale® DDS can be positioned in the inspiratory limb of the breathing circuit or is equally suitable in a catheter mount or connected directly to the patient connector. Spirale® is intended to remain in the breathing system and does not require disconnection from the breathing system for each treatment, reducing the risk of ventilator acquired pneumonia (VAP). During invasive mechanical ventilation a chamber spacer with a pMDI was up to sixfold more efficient for aerosol delivery. (2)
Effective bronchodilator therapy is pivotal to successful stabilisation and weaning of mechanically ventilated patients. (1)
During mechanical ventilation, larger particles are trapped in the ventilator circuit and endotracheal tube. Devices that produce aerosols less than 2μm are more efficient. (2) A spacer reduces the velocity of the MDI plume and allows evaporation of the propellant, which increases drug delivery.
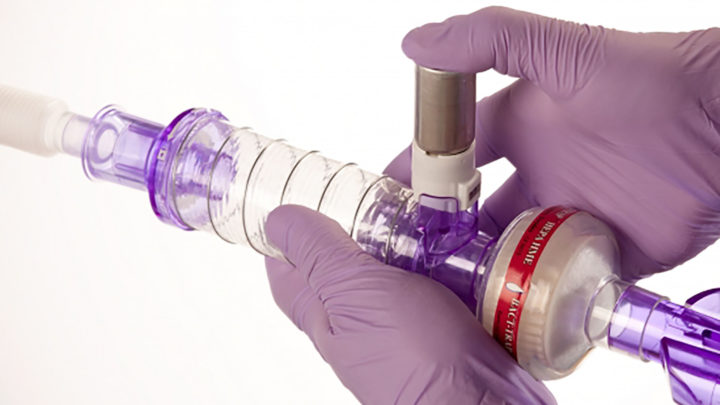
Non-Invasive Ventilation
Patients with acute respiratory failure are often removed from non-invasive ventilation to receive inhaled bronchodilators. (4) Many patients may not tolerate removal of their respiratory support and subsequently their bronchodilator therapy may not be completed.
Patients may be adequately oxygenated while receiving NIV but if removed, even for a short period, catastrophic hypoxia may occur.”
Effective bronchodilator therapy is pivotal to successful stabilisation and weaning of mechanically ventilated patients. (1)
Administer Bronchodilators with Spirale® DDS for minimal disruption to the patient’s respiratory support.
Spirale® enables continued respiratory support during bronchodilator therapy.
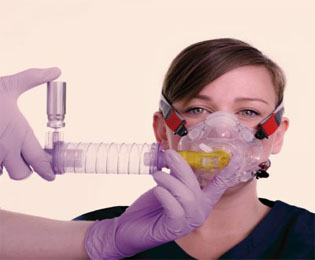
Product Specifications
- Optimal drug delivery
- Universal actuator port
- Compatible with MDIs with dose counters
- Rotational locking system
- Self-expanding when unlocked
- Low (16ml) dead space when closed
- Eliminates environmental drug pollution
- Maintains a closed breathing circuit
- Avoids disruption to ventilation settings
- Does not require additional oxygen